SIQ
Social activities at SIQ: snapshots from the May picnic
This year again, we organized a traditional SIQ picnic in a lovely picnic place in the vicinity of Ljubljana. The picnic offered guided sports and an entertainment program with a competition in a team spirit. Seven teams competed in five disciplin...
Find out more
Complete Solutions for Medical Devices
We provide support to manufacturers of medical devices all the way from the idea and design to the placing of the device on the market with a variety of services – we train, test and certify. The comprehensive range of services...
Find out more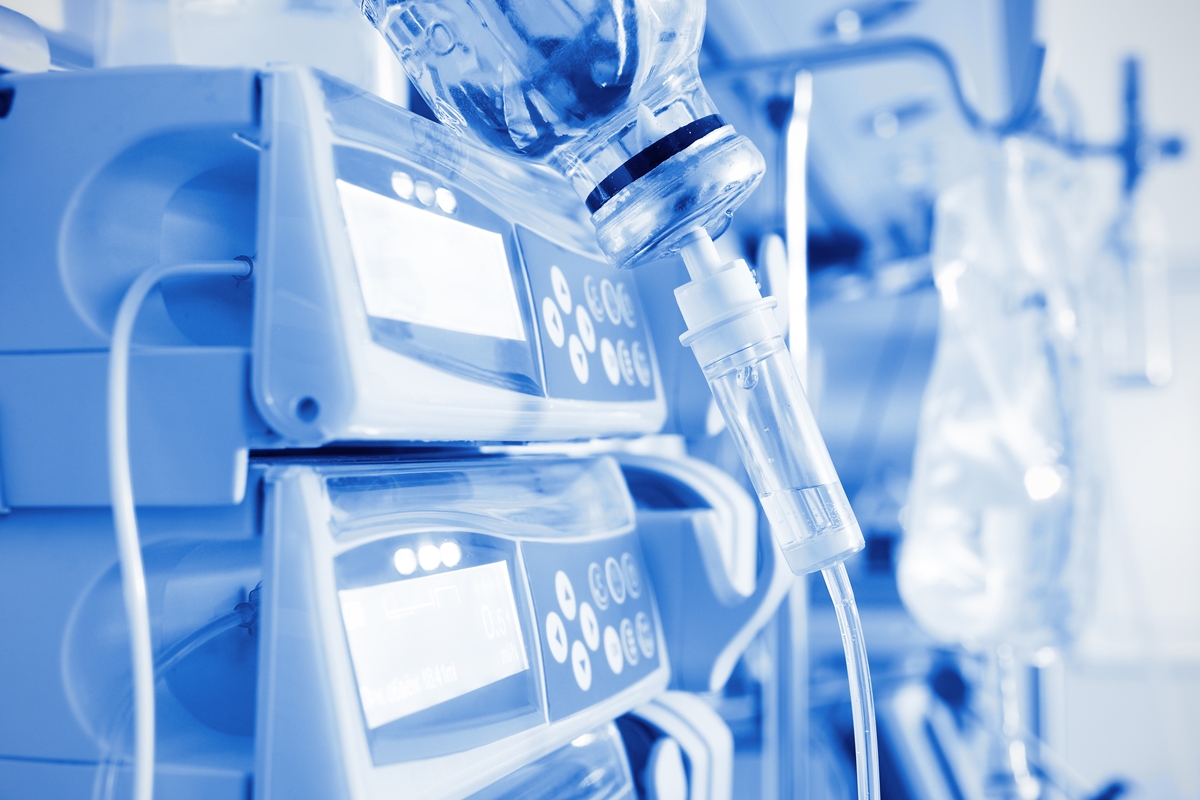
Complete solutions for Medical Devices in Accordance with the Medical Device Regulation
Medical devices play a key role in saving lives. They account for more than a quarter of the world market. This is a sector that is constantly and rapidly evolving and that requires constant adaptation of all stakeholders. The human population is ...
Find out more
7th International Safety & EMC Training Seminar
On May 12th and 13th, we successfully hosted our 7th International Safety & EMC Training Seminar at Hotel Kempinski in Portorož. More than 115 participants from 18 different countries joined us at the seminar, where they received an in...
Find out more
SIQ and GLI in Final Discussions on Strategic Cooperation
We are very pleased to announce that SIQ Ljubljana and GLI, the leading global provider of the gaming industry’s testing and certification services, are in final negotiations to cooperate in the field of gaming technologies. We strongly believe th...
Find out more